how to find formal charge
Formal charges in organic chemistry is, perhaps, one of the most fundamental bookkeeping devices which is often misunderstood or neglected by students.
Why Formal Charges are Important in Organic Chemistry?
Knowing formal charges can help us understand the reactivity patterns in reactions, find reactive centers, and make sense out of electron flow in the mechanisms. For instance, negatively charged species tend to be the sources of electron density in reactions, while the positively charged species—accept those electrons.
Here is a couple of examples of molecules with formal charges:
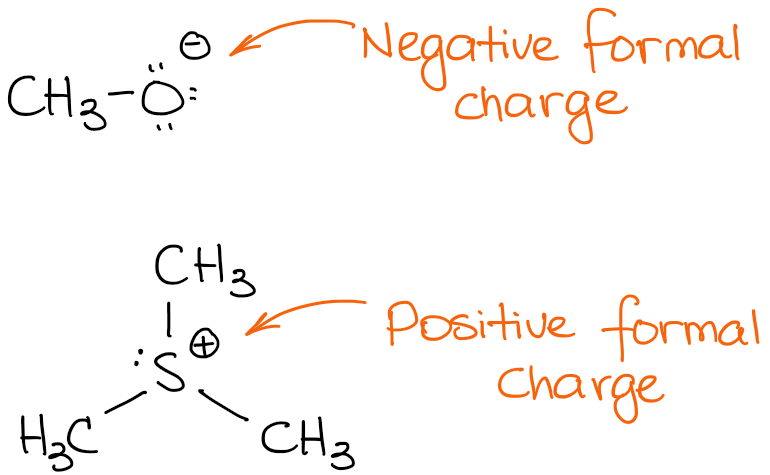
The top species has a negative charge. We call such species anions. Since it has a negative charge, it means that it has an excess of electron density. Thus, it is likely to be the source of those electrons in a reaction. Sources of electron density in an organic reaction are acting as nucleophiles or bases.
The second species bears a positive charge, thus it is a cationic species. In a reaction, a cationic species will be an acceptor of electrons acting as an acid (either Brønsted or Lewis) or as an electrophile.
It's important to keep in mind that a formal charge is not the same thing as an actual charge! I'll talk about it a little later in this post.
How to Calculate a Formal Charge
The "official" way is to subtract 1/2 bonding electrons and nonbonding electrons from possible valence electrons that an atom can have. In other words:

What are all those terms?
Bonding electrons are those that make up bonds. Each bond contains 2 electrons. So, 1/2 of bonding electrons equals to the number of bonds and atom has.
Nonbonding electrons are those that are not participating in any bonding. In other words, nonbonding electrons are the spare electrons (usually electron pairs) on an atom. Using the examples from above we have:
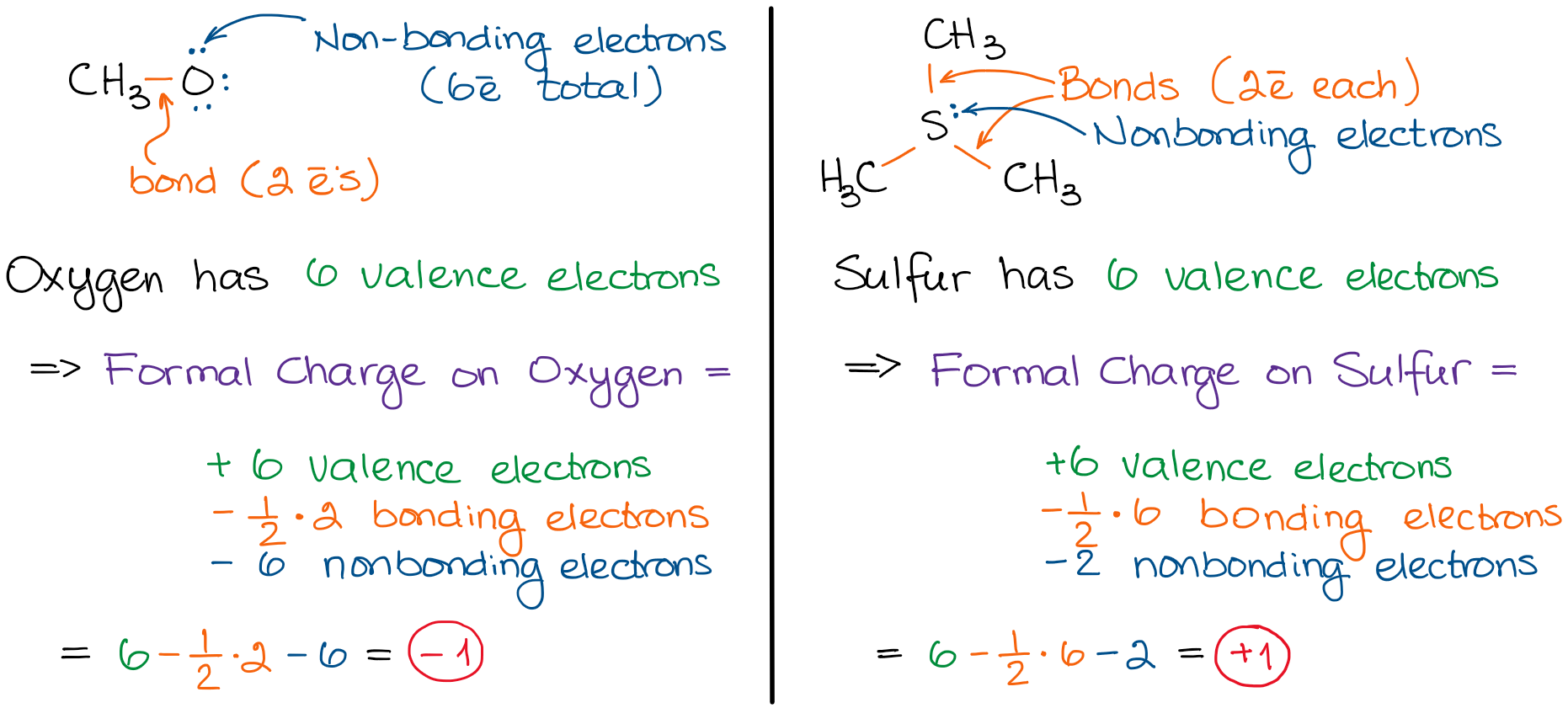
The definitions and the "official" method looks a little ugly. As a professional chemist I can talk all day about the "official rules" and "proper names" and bore you to death. Instead, I'd rather you use a simple "trick" that always works and, essentially, is the same thing. The trick is:
Formal charge = Valence Electrons – Sticks – Dots
The number of valence electrons equals to the element's group (column) in the periodic table. This way, carbon has 4, oxygen has 6, and hydrogen has 1 valence electrons. The bonds and the spare electrons will be indicated (or can be easily found from) the molecule's Lewis structure. So, for as long as you have a complete Lewis structure and periodic table handy, you can quickly find the formal charge of any atom in a molecule.
The Difference Between the Formal and Actual Charge
Now, I've mentioned earlier that there's a difference between the formal and the actual charge. Formal charge is a bookkeeping tool that is important to help us keep track of the electron flow in the reaction.
The actual charge, however, is the actual electron density that is present on the atom. For instance, let's take a look at borohydride anion:

The electronegativity of boron is 2.0 while electronegativity of hydrogen is 2.2. So, the hydrogen is more electronegative (not by much but still) and will polarize the bond. This means that hydrogen actually "pulls" the electron density towards itself. Thus:
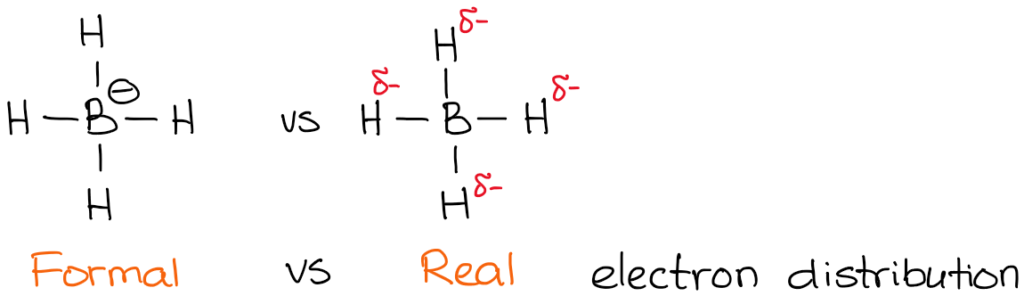
While formal charges are merely a "formality," they are very important for the reactions mechanisms understanding. Thus you need to make sure you master the skill of quickly finding the formal charge.
You also notice that I've indicated my real electron densities with the delta-minus (𝛿-) symbol. That denotes that I only have a partial negative charge on each of the hydrogens. How much of that partial charge we have on them? Well, we could calculate it using fancy quantum chemical calculations, but that's utterly unnecessary for the purposes of a typical organic chemistry course. What's more important, is to realize that the boron is not actually negatively charged in this molecule. So, when we write a reaction with a borohydride anion, we won't be showing electrons coming from boron!
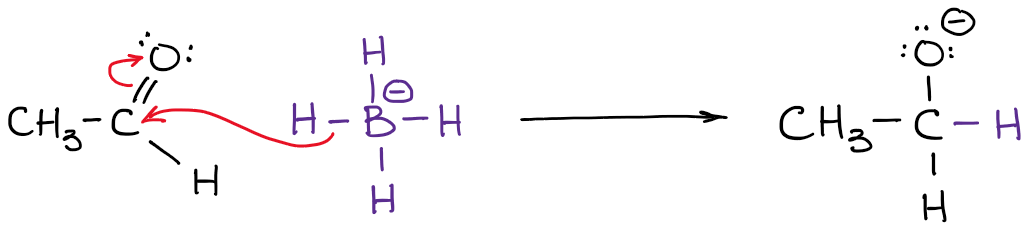
Notice how in the example above my arrow starts at the H-B bond and not at the boron atom! That's because it's not the boron that is a source of electron density here! You remember from earlier in this post, it's the hydrogens are the atoms with the 𝛿-. So now it makes sense that the arrow doesn't start at boron.
how to find formal charge
Source: https://www.organicchemistrytutor.com/lessons/formal-charges/
Posted by: usherseentacts.blogspot.com

0 Response to "how to find formal charge"
Post a Comment